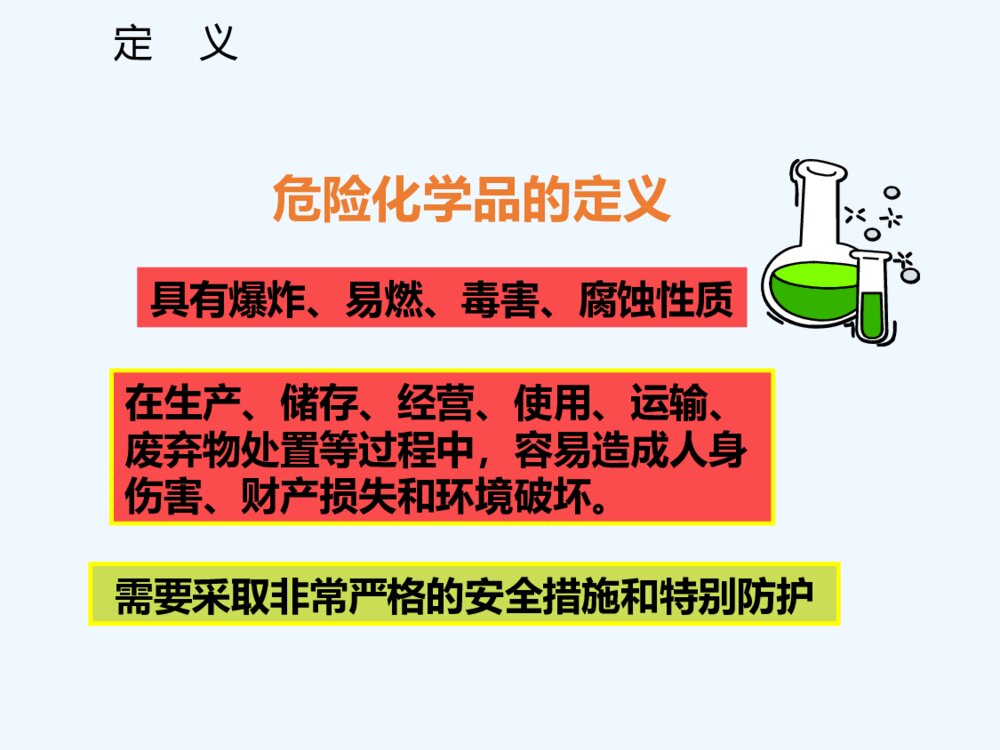
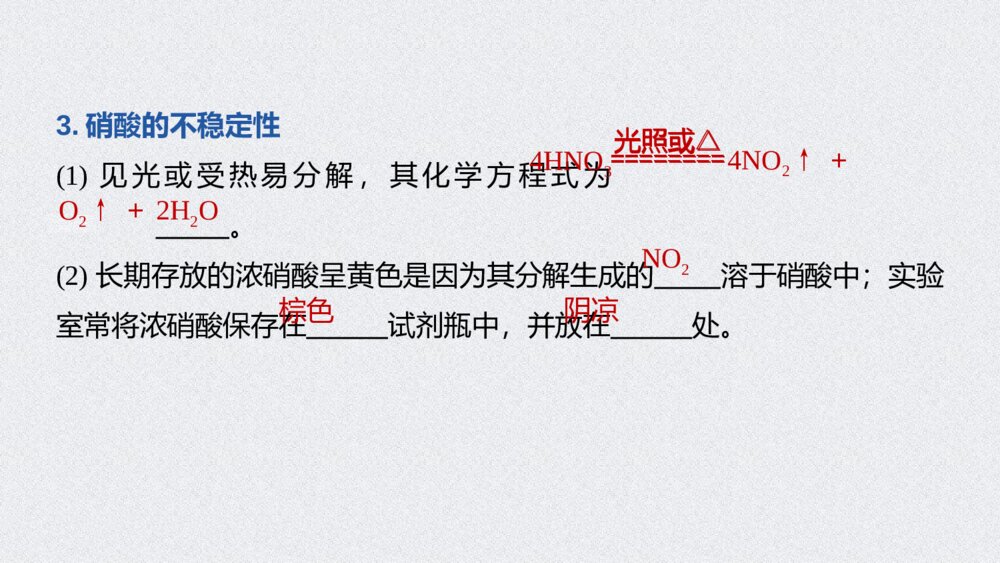
《氮及其化合物 硝酸 酸雨及防治》人教版高中化学必修二教学PPT课件




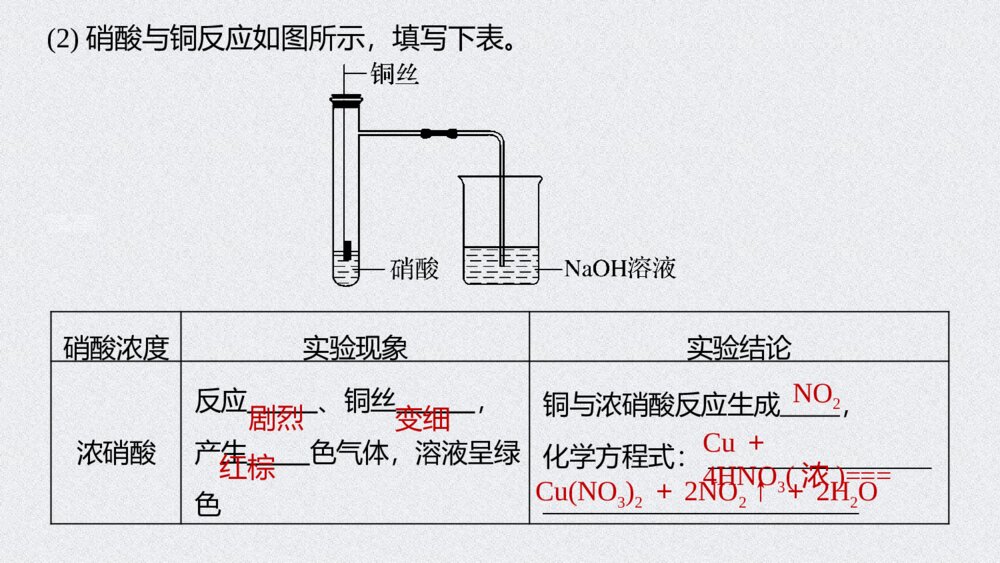
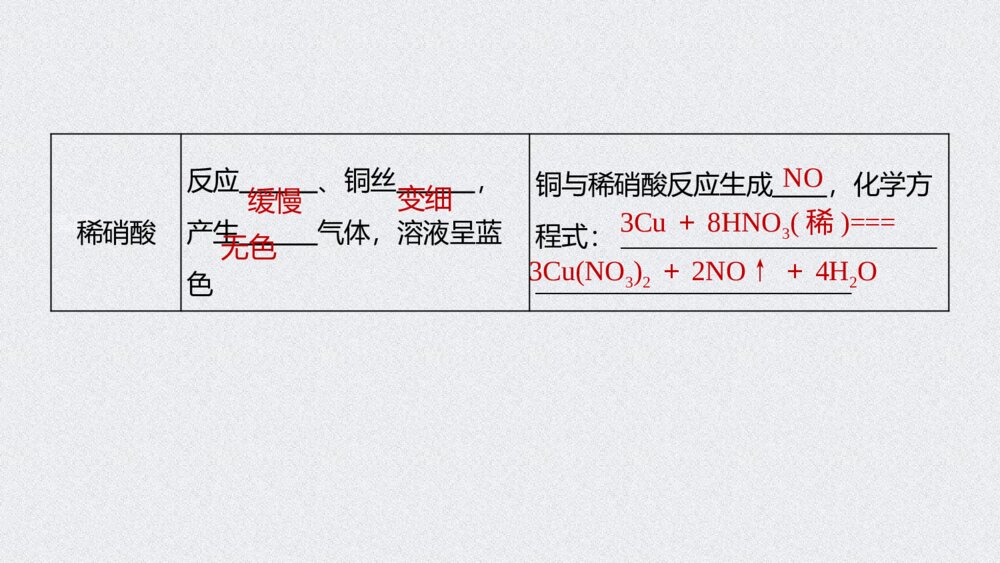


试读已结束,还剩25页未读,您可下载完整版后进行离线阅读
《《氮及其化合物 硝酸 酸雨及防治》人教版高中化学必修二教学PPT课件》是由用户上传到老师板报网,类型是化学课件,大小为600.69 KB,总共有35页,格式为pptx。授权方式为VIP用户下载,成为老师板报网VIP用户马上下载此课件。文件完整,下载后可编辑修改。更多关于请在老师板报网直接搜索
- 页数:35页
- 大小:600.69 KB
- 编号:12034
- 类型:VIP资料
- 格式:pptx
- 提示:数字产品不支持退货